- Certification >
- Medical Device Certification >
- NSAI Medical Device QMS and/or CE Certification Process
NSAI accepts applications for certification in English only. This is also the only language offered in terms of communication and on site quality system auditing.
The steps involved in obtaining QMS and/or CE certification are:
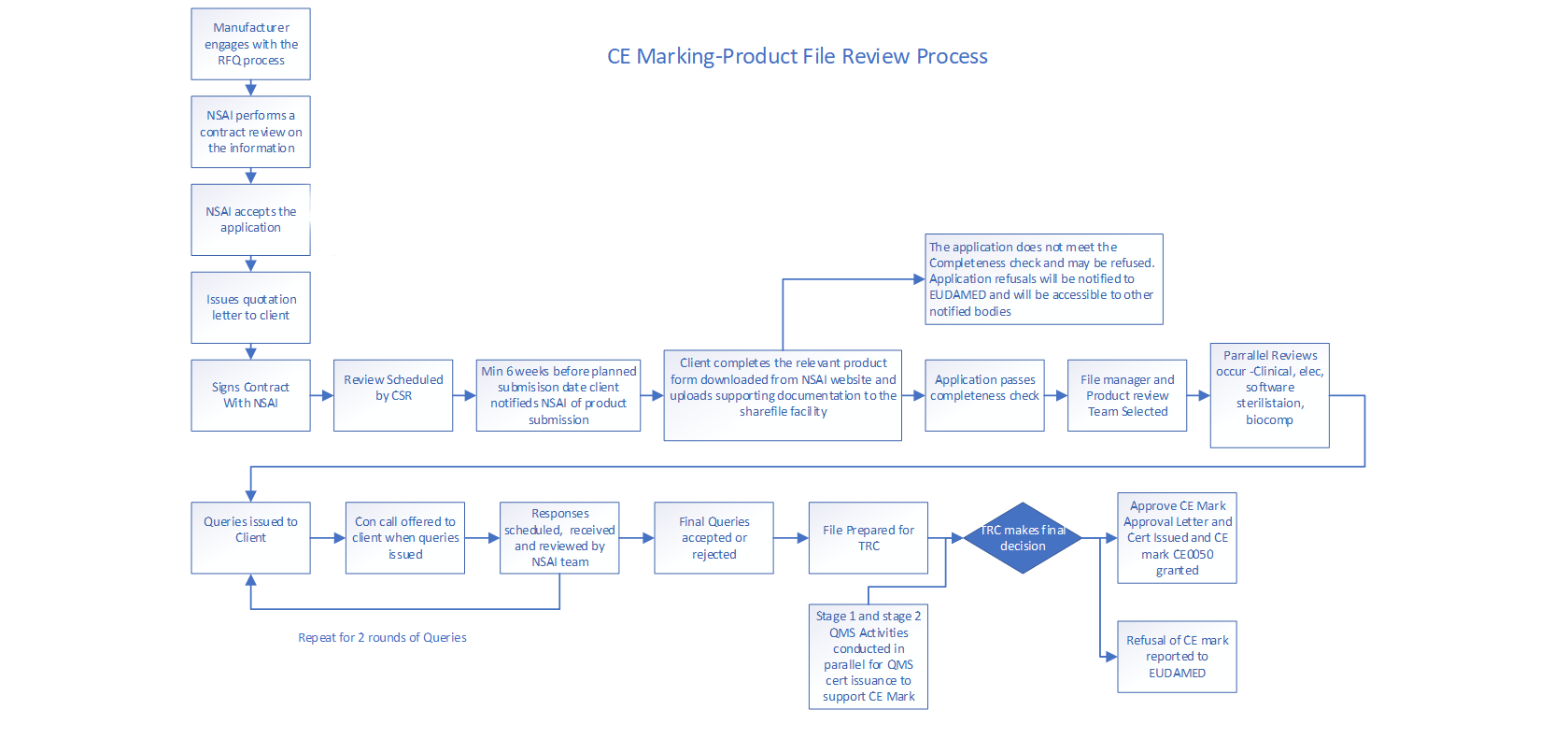
- An Application/Request for Quotation form is downloaded from the NSAI website and submitted per the instructions on the form.
- NSAI reviews the Application/Request for Quotation and conducts a contract review.
- If the contract review is successful, an NSAI quotation letter is sent to the applicant in conjunction with Schedule 1 - General Terms and Conditions, and Schedule 2 - Special Terms and Conditions.
- If the contract review is not successful, the applicant will be informed.
- If the applicant agrees to the quotation letter, they are required to sign and return a copy to NSAI.
- The contract is now in force between NSAI and the applicant. Quality system audits and, where applicable, technical documentation reviews can now be scheduled and conducted.
Quality Management System Audit Process
Stage 1 Audit Assessment
- The NSAI Lead Auditor requests relevant documentation for review by issuing the preliminary audit report.
- For MDR 2017/745 and IVDR 2017/746 Stage 1 audits, the manufacturer is required to complete a technical documentation questionnaire.
- In general, a portion of the assessment will occur onsite at the Client’s location.
- If applicable, open dated invitation letters for Visas are required to be provided by the Client to the audit team to allow visits to sites, suppliers and sub-contractor locations.
- NSAI will review at least the following documentation:
- The scope of the quality management system
- The quality management system documentation
- Internal audit and management review
- Legal and regulatory requirements
- Customer specific requirements
- This list is not exhaustive and is subject to change. - At the close out meeting, NSAI will provide an audit report. This may or may not contain non-conformances with the applicable Scheme/Standard/Directive/Regulation.
- NSAI will then determine whether to proceed to stage 2 audit assessment.
Stage 2 Audit Assessment
- NSAI will provide an audit plan and identify Audit Team members in advance of the audit.
- An opening meeting between the Client and NSAI Audit Team will be held on Audit Day 1.
- A facility tour may be requested.
- NSAI will conduct a comprehensive on-site assessment of the quality management system.
- Throughout the assessment, the NSAI Audit Team will have regular meetings with the Client to review progress and allocate resources.
- If it becomes apparent during the audit that the Client is not ready for registration, the Lead Auditor will organise a meeting with the Client’s senior management team to advise them of the situation.
- The NSAI Audit Team will analyse the data gathered from stage 1 and stage 2 audit assessments and will determine the audit conclusion.
- A technical review of the audit documentation is performed.
- Upon NSAI satisfaction with the technical review, the audit documentation will be presented to the NSAI Certification Review Committee.
- Subject to NSAI’s positive determination, Certification will be granted.
Surveillance audits
Following the initial certification audit, the first surveillance audit typically occurs within 6 months of certification.
All subsequent audits will be conducted on an annual basis for ISO 13485 and MDSAP, and at least every 12 months for MDR and IVDR.
3-year reassessment audit
Except in the case of MDR and IVDR, a reassessment audit is conducted at 3-year intervals.
This will include the review of previous surveillance audit reports. If there is a positive audit conclusion and if audit documentation is deemed satisfactory by the NSAI certification review committee, continued Certification will be granted.
Unannounced audits
Unannounced audits will be conducted within the Certification cycle. An unannounced audit cannot be refused and can be extended to critical suppliers and sub-contractors.
For cause or special audits
For cause or special audits can occur within the Certification cycle and can sometimes occur at short notice. For cause or special audits cannot be refused and can be extended to critical suppliers and sub-contractors.
CE Marking Process for Product Certification